Lipid peroxidation is a well-known mechanism of cellular damage in both plants and animals and is used as an oxidative stress indicator in cells and tissues. Lipid peroxides are unstable and are rejected to create some elaborate compound sets, including reactive carbonic compounds. Polyunsaturated peroxide (MDA) and 4-hydroxyalkyl (Hae) dehydrated acids. Measuring malondialdehyde and 4-gidroksilalenov used as a marker of lipid peroxidation (1). This method is meant to test MDA (hydrochloric acid) or MDA-4 gidroksilanami (methanesulfonic acid).
Materials available
o Reactive R1
or Reactivity R2
o MDA Standard
thin
1 x 16 ml of 1,1,3,3-tetra methoxy propane in Tris-HCl (1 x 1 ml Demir Iron, 3 x 18 ml of methanesulfonic acid (MSA), N-methyl-2-phenylindole ml
Required but did not provide materials
- o Spectrophotometer for absorbent measurement at 586 nm from measurement units 0-2.
- o Spectrophotometric cuvettes with 1 cm optical path length.
- o Install a water tank to control the temperature at 45 + 1 ° C.
- o Vials and disposable glass plugs that are compatible with acetonitrile, methanol, and acid.
- or HCl, 37%
“Hydroxyethyl Butyl (BHT)
“Acetonitrile
“Microcentrifuges
“Acid-resistant micro-magnetic tubes / acetonitrile (eg, polypropylene).
Warnings and Precautions
“Do not smoke, do not eat or drink in places where samples and reagents are processed.
“Wear disposable gloves, samples, and reagents.
“Do not add piped reactions or samples in your mouth.
“In the case of accidental exposure to R1 and R2 reactions to the skin, mucous membranes or eyes wash well on the surface with water.
“In vitro use should only be used for research purposes in diagnostic methods.
Reagent Storage and Handling
“Do not leave the reagent bottle as soon as it breaks what is needed.
“Good practice is the amount of reagent needed to transfer the experiment to a clean glass vial or another boat and adjust reaction bottles to 4 ° C.
“Do not allow your closed reaction bottles to be run at room temperature for a long time, or use bottles at 4 ° C.
“New pipes or pepper tips should be used when reagents are being transferred from vials, without avoiding contamination of vials, as this may affect the results of the analysis.
“If the reagents are processed and stored correctly as described above, they are stable until the expiration date.
Methodology
1. Prepare the reagent
Lift solution R1 for use in tsayay. Add one volume (6 ml) of a knob, by size (18 ml) of reagent R1. Prepare this solution just before using it. Do not leave the reagent bottle R1 without the cover (open the atmosphere to open).
HCl Preparation 37%
HCl is 37% about 12 N of an acidic reagent. Here is a concentrated HCl power in many chemical companies. Do not pollute HCl before you use it.
Sample preparation
Note: Before starting the sample preparatory procedure, read the relevant parts of the NOTES.
Tread propaganda oxide
It is recommended that the BHT investigator with a final concentration of 5 mm in a buffer before homogenizing tissue or cells. BHT can be done in acetonitrile in the form of a solution of 100% of a solution of 0.5 M. If no antioxidant is added, new lipid peroxide can occur during homogenization, and there will be offset values
(2). Homogenate-sensitive tissue / tissue
Examples of homogenates should be targeted as much as possible. The concentration of protein in the homogenate should be determined. In a single biological sample, 0.2 ml of homogenate is recommended with 15-60 mg/ml of protein for initial studies. For tissue culture cells, it is recommended that the sample obtained from 107 cells was added to the test (i.e., 0.2 ml per ml, 1 × 107).
Sample Stability
If this was not done immediately, the samples should be frozen at -70 ° C to prevent the loss of MDA and 4-hydroxylamines (HAE) (3,4) and prevent new sample oxidation. Samples should not be stored at -20 ° C. The sample used in the fridge should not be dissolved when stored at -70 ° C for testing. To avoid photo-oxidation, the samples must be protected from light.
Sample preparation procedure
Homogeneous tissues
1. If necessary, do in vitro blood through in situ perfusion with an isotonic willow solution or rinse it with isotonic cold saline (i.e. 0.9% NaCl).
2. Cloth
3. Preparation of tissues in buffer 20 mm phosphate (pH 7.4). Another buffer can also be used, but the investigator must eliminate the abolition of the MDA dilution standard in the selected buffer.
4. Add ten μl of 0.5 M BHT in 1 ml of homogeneous tissue in acetonitrile to oxidize the sample.
Precipitation is expected. It will be centrifuged, and the results will affect.
Experiment.
5. Make the homogenate of its centrifuges to remove large particles (e.g. 3000 x g, 10 minutes at 4 ° C).
6. Remove some of the samples to identify the protein.
7. Immediately run the sample at -70 ° C or leave it on ice before it is tested. Test 0.2 ml of homogenate
During the tests.
Cell Culture
The protocol is similar to the prototype homogenate tissues. However, it is advisable to prepare homogenate 5 X 107 cells per ml for use in the away. Spouses should be paid their culture into a serum meter with some boring components before homogenization.
Plasma or serum
The amount of MDA or HAE is free in normal plasma or serum below or below the lipid transfer test detection limit.
Prepare standards
Malondialdehyde is shown as acetyl since the aldehyde itself is not stable. The acetyl acid (TMOP) is hydrated at 45 ° C. during the construction phase, and MDA is obtained.
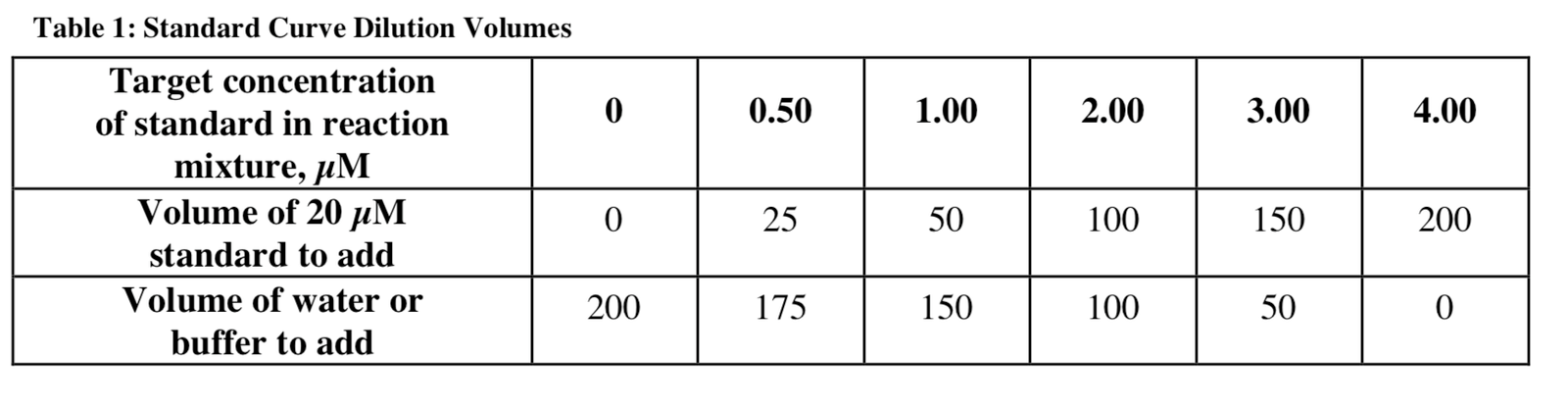
The TMOP standard is provided as a 10mM stock solution. Go to 1/500 (v / v) Water just before using a 20 μM stock solution for use in the experiment. About a standard pipe curve, the amounts shown in Table 1 total 200 μl of quality.
ASSAY
MDA analysis procedure (dissolution of hydrochloric acid procedure)
1. Prepare standards in Table 1 for clean glass vials or polypropylene microprorphine tubes. Of course, the standards must be met three times.
2. Add a 200 μl sample with a clean glass vial or a micro-water tube made of polypropylene. Unknown must be run as a triple.
3. Add 650 μl of diluted reagent R1.
4. Delete the sample smoothly.
5. Add 150 μl of 37% (12 N) HCl.
6. Mix the tube and stop it.
Go for 60 minutes at 45 ° C
8. Obtain clear fibers through the transparent samples of its centrifugal (e.g., 15,000 x g, 10 minutes).
9. Transfer the transparent hospital to the dish.
10. The measure of the execution at 586 nm. *
The experimental procedure for MDA + HAE (MSA dissolution procedure)
It is recommended that the MDA be used as a standard when testing the size of MDA + HAE. Multiple studies that report MDA and HAE-free concentrations show that MDA is about ten times more. Since the slopes of the calibration Aids for MDA and HNE are not equal, there are no inevitable errors when both carbides are recognized. However, the standard MDA curve will reduce the error the worst.
1. Preparation of standards according to Table 1 in clean glass vials or micro-receptor tubes from polypropylene. Of course, the standards must be met three times.
2. Add a 200 μl sample with a clean glass vial or a micro-water tube made of polypropylene. Unknown must be run as a triple.
3. Add 650 μl of diluted reagent R1.
4. Delete the sample smoothly.
5. Add 150 μl of R2 reagent.
6. Mix the tube and stop it.
Go for 60 minutes at 45 ° C
8. Obtain clear fibers through the transparent samples of its centrifugal (e.g., 15,000 x g, 10 minutes).
9. Transfer the transparent hospital to the dish.
10. Absorption is measured at 586 nm. *
* The room temperature is at least a temperature for at least one hour, 2 hours at 4 ° C if it is stored in the dark and there is no rotation.
Sample Blank (Asb)
The void sample should be measured to correct any contribution from the A586 due to the sample. This space is made by adding to the test tube the 650 μl of 75% acetonitrile / 25% dilute instead of the diluted reagent R1. Then, the steps related to the acids and the sample are fed up as described above.
Empty Jet (Ao)
Tablada has a reactive void in 200 ml of water, which determines quality preparation.
Standard curve properties
In HCl and MSA, the molar loss (e) coefficient of approximately 120,000 is for the 586 nm formaldehyde. The color result is a linear function of the MDA concentration in the range of 0 μM to 20 μM.
4-HNE cannot be measured in the HCl protocol since it is not a reagent with a reagent when HCl is an acidic solvent. The MSA procedure should be used to determine MDA and 4-HNE at the same time. The moisture coefficient used by MSA is determined indirectly from the slope of the standard curve, similar to the amount described in the sample.
Conclusion and Discussion
The smallest concentration is acceptable.
Experiments on standard entaldegida voids show that the analyzed detection limit in the purified system is 0.1 nmol / final concentration of ml (0.5 nmol/ml in the sample) and corresponds to the absorption value of approximately 0.011. The limit for biological samples will be higher, and researchers are encouraged to evaluate these parameters in their systems.
stability
Experiments containing standard samples (0-20 .mu.M) Testing for ten days using the protocol, based on standard measurement error (SEM) is less than 5%.
Analysis of lipid peroxide on microwave plates
For those interested in applying the lipid transfusion test to microtitre plates, we provide the following information.
1. The test for lipid peroxide is not tested on microwave plates.
Two must be performed in closed vials/microcentrifuge for 45 ° C incubation and mixing of the sample (e.g., centrifugal) sample, minimal loss due to sample haul, and successful clearance.
3. A dimension of absorption of clear samples in microtitration plates:
1. The deprivation coefficient for the MDA / 4-HNE will depend on the length of the active path in a certain microtiter plate reader.
2. According to the concentration, enaldegida total absorbance would be low, and the sensitivity of the assessment will be reduced.
3. If continuous monitoring wavelength is available, samples for measuring filters must be 580 nm or 590 nm.
4. The user must determine that the certain microtiter plate used chemical acetonitrile is very acidic resistant.
4. The amount of the reaction mixture: it is necessary to use at least 0.5 ml of a total reaction mixture to ensure that the 0.2-0.3 ml of the sampled sample will determine the absorption.
5. When obtaining the conditions to measure clear samples of microplate plates, they should be applied simultaneously.