Introduction
The mention of saltwater or more commonly referred to as saline water, raises the general question every individual may have thought of, at some stage of “Is saltwater is drinkable?” or “Why is saltwater undrinkable?” Water encompasses about one-third of the earth’s total surface. Fresh drinking water forms about 1 percent of it, whereas two to three percent belongs to glaciers while the remaining amount, most compromising of saline water, forms the oceans. The fact of saline water being undrinkable is related to kidneys in the human body and the way they process water. The reason resides in the fact that drinking salt water will evidently result in dehydration and unfavorable circumstances arising due to it.
However, presently there are methods through which saline water or saltwater can be processed to a fitting drinkable state. Since the freshwater deposits are slowly decreasing and the level of water is dropping at a gradual pace, research into turning saltwater into drinkable water has been seeking out ways to make it available for masses. However, since desalination requires large amounts of energy to convert for the process, and in order to carry out the needed task facilities where such a process. Plants required for desalination of saltwater and demand a hefty amount of funding to carry out the process. As such, governments are less likely to adopt this method to resolve the crisis of diminishing fresh water reservoirs.
Discussion
The human body, constituted of about 50-60 percent water, exclusively relies on its continuous intake. Most of the water acquired for human consumption comes directly from fresh water reservoirs. However, Earth’s surface forming up to one-third of water consists of about ninety-seven percent salt water, also known as saline water. Saltwater, being in an undrinkable state, poses a threat to humans. The process of desalination exhibits the process in which salt water, which is naturally heavy in density than fresh water, is slowly boiled till the water droplets gathered at the top of the flash can be removed/collected. These droplets are salt free and fit for the use of either cooking or drinking and will pose no such threat to the human body.
Figure 1 Salts in Water
The above picture displays a detailed level of salt in seawater, exhibiting the relatively higher levels, compared to salt levels in fresh water. The human body will show signs of cell damage if any large amount of fluid, with higher levels of salt. The cells will start showing signs of crushing inwards and eventually dying.
The objective for this is to make salt water more drinkable since the earth is 70 % water and in that water, only 3% of the earth has fresh water the other 97% is salt water which is very unpleasant and unhealthy. So to make the salt water drinkable, there are many methods. The easiest and widely used process to create saltwater drinkable is to boil the salt water. In this way, water evaporates at 100-degree centigrade, and then evaporated water vapors are collected and cool down to get pure and drinkable water. Whereas the salt doesn’t evaporate at 100 degrees and remains in the flask. Hence in this way water and salt are separated from each other. The below pie diagram shows the amount of salt present in salt water, Note that in 1 kilogram of water, 34.4 grams of salt is present(Can You Make Seawater Drinkable? | APEC Water).
Figure 2(Salty Oceans)
The above pie chart gives out a detailed explanation of salt water composition and its chemical constitution. This indicates that to live, we must be hydrated at all times and drink water. In other words, our lives depend on keeping our bodies hydrated in order to survive. Water essentially forms as one of the most fundamental requirements for our body. Water, because of its quality of being a good solvent, is essentially important for almost all life on Earth. The process of turning salt water can be performed by having the following material and following the below-mentioned steps in a precise manner,
Materials
- Water
- Salt
- A stove or a Bunsen burner
- A glass distilling flask
- A drinking glass
- A 4-quart saucepan
- A large leaf from a non-toxic plant (banana, fig, or taro are good choices).
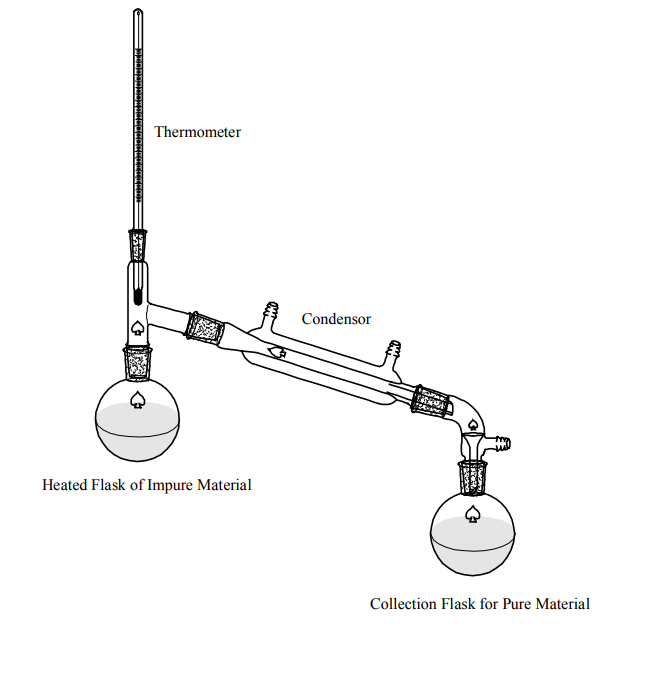
Figure 3 (Rives and Riley)
STEPS
- Adding 70 grams of table salt in 2 liters of fresh water
- Take some fresh water in a flask
- Add some salt to the water and mix it gently to mix it well.
- Taste the salt water and be sure to spit it out. ( Don’t drink it)
- Write down the saltiness of the water tasted and rate it on the table below.
- Smell and record the scent of the salt water.
- Write down the current look of the water in the table below.
- Take another empty flask and connect the two flasks with the help of a condenser as shown in figure 3.
- Also, place the thermometer in the flask that contains the salt water
- Now heat the flask which contains salt water and note the temperature on the thermometer
- Heat the flask gradually until the temperature reaches up to 100-degree centigrade
- At 100 degrees, the water in the flask starts to evaporate because the boiling point of water is 100-degree centigrade.
- The water vapors come out from the flask and move to the other emptied flask, and the condenser cools down the water vapors to convert them back to the liquid state.
- Salt will remain in the heating flask since salt doesn’t evaporate at 100-degree centigrade.
- In this way, water and salt will become seperated from each other and water can easily be collected from the vapors forming at the top.
- Water that is collected in the other flask is 100% pure and drinkable
- Based on the outcome, rate the distilled water.
- Smell the water and record the current smell of it.
- Observe the water state and note it down.
Results
Once the experiment is complete, a close analysis of the process will explain the process it takes to separate the salt from water. The water extracted from this process is pure and drinkable with the un-tasteable amount of salt present it. This method is used primarily in the Middle East. Kuwait and Qatar rely on this technique to extract drinkable water for their use. While some countries use this way to extract salt from the water and use the salt for cooking food and other uses.
| Taste (saltiness) | Smell | Sight | |
| salt water | 8 | salty | Clearwater |
| fresh water | 1 | No smell | Clearwater |
| distilled salt water by flask | 2 | No smell | Clearwater |
Once the readings have been properly noted in the table above, the significance between the two can be seen quite clearly. Distinguishing the difference between a sample of salt water and fresh water. Being different in their smell and taste, it will become evident towards why we can’t drink saline water. Seeing how the saltiness for salt water is at 8, it shows fresh water ideally more favorable towards drinking. However, distilled salt water acquired after the process of separation of salt, is a favorable option after fresh water. Although, the column labelled “sigh” signifies that both salt water and fresh water look alike and will be hard to identify between the two from simply observing them.
Conclusion
Conclusively it can be stated that salt water, in a processed form, can be turned into a drinkable state. The process of desalination requires a series of steps to be followed, as stated in the discussion above. Performing the steps carefully can assure the possibility of minimizing the levels of salt levels, to a drinkable state. After boiling salt water, it evaporates at 100-degree centigrade, and then evaporated water vapors are collected and cool down to get pure and drinkable water. However in the case of salt, it doesn’t exactly evaporate at 100 degrees and remains in the flask. In this way, it is possible to collect water while keeping the salt separated.
Presently, the desalination process is being carried out in California to cover up the need for water for the local residents. However, desalination requires massive amounts of funding and as such can pose a financial threat for the government. The process of desalination in the above-mentioned steps signifies the process of performing the desalination process in a controlled environment. Boiling salt water to 100 degrees centigrade will cause water to start evaporating while leaving the salt behind. The vapor can then be collected, making it drinkable and safe for consumption. This process can also be conducted by boiling water at lower levels of heat.